FDA Approved Mentor® Cohesive Silicone Gel Breast Implant
You may have read Dr. Elliott’s previous blogs about Sientra and Allergan’s FDA approval of cohesive silicone gel filled breast implants over the past year. Recently, the Food and Drug Administration (FDA) has approved a third highly cohesive silicone gel filled breast implant manufactured by Johnson & Johnson unit, Mentor Worldwide LLC.
As with the other FDA approvals of cohesive silicone gel implants, these Mentor® implants underwent rigorous testing and review. The FDA collected six years of data from 955 women to ensure safety and effectiveness of the MemoryShape Breast Implant.
Some believe that these silicone gel implants may provide firmer, more natural-looking results compared to other implants. Similar to other implants, they come in different sizes and textures to fit your unique body shape. During breast augmentation consultations, Dr. Elliott discusses different breast implant options to determine which will best benefit the patient’s aesthetic goals and desires. Patients with cohesive gel-filled implants have the advantage of better maintaining their shape after trauma or rupture.
As with all cosmetic or reconstructive surgeries, Dr. Elliott encourages his patients to thoroughly research and discuss their surgical options with a board-certified plastic surgeon.
For more information about breast augmentation surgery or other procedures Dr. Elliott offers, please contact us to schedule a consultation. Connect with Dr. Elliott on Facebook, Twitter, and Google+ for the latest plastic and reconstructive news.

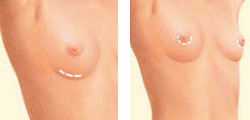 Breast enlargement surgery
Breast enlargement surgery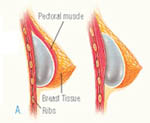 Regardless of the type of implant you receive, it’s important to follow up with your breast surgeon regularly and undergo annual mammograms with technicians who have experience screening women with breast implants.
Regardless of the type of implant you receive, it’s important to follow up with your breast surgeon regularly and undergo annual mammograms with technicians who have experience screening women with breast implants.